
WE are now one quarter down since the publication of the MDR & IVDR and it is time to think about your compliance strategies and start making plans as to how you might deal with all the changes required, including those for your quality management system.
And, in case this is not enough – you also need to maintain regulatory compliance and CE marks for your individual devices.
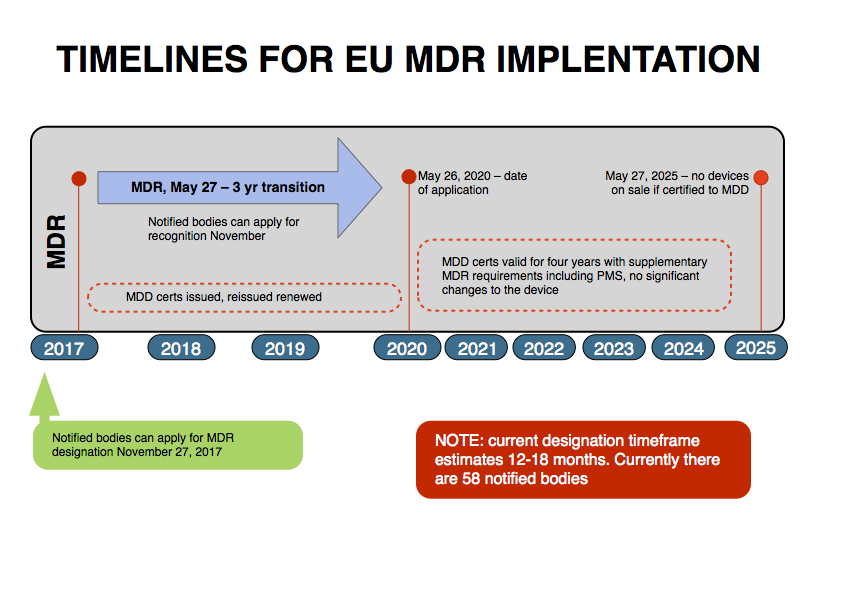
We are aware of the published timeframes, and the outline below should help to reinforce that message and understanding.
Every stakeholder will be under significant time pressure ie the manufacturer, the Notified Body and the Competent Authority.
For those of you who have read the MDR, you will know the NB’s cannot apply for designation until six months after Entry into Force.
The designation process is currently estimated for 12 to 18 months duration.
Although there are 58 NB’s, there is recognition that not all of them will be designated at once and discussions as to how best can this be managed.
As you consider your regulatory strategy, which is a requirement of the MDR, it is important that you take account of the timeframes in relation to the expiry dates of your certificates for both your QMS and CE Marking.
Individual device product lifecycles should help you to identify the additional work which will be required to construct technical files/design dossiers to meet the requirements of the MDR.
Your actions, plans need to have started.
E-mail:
Tel: +44 (0) 141 946 6482
Address: Healthcare Skills Training International Ltd
West of Scotland Science Park
Block 7, Kelvin Campus
Glasgow G20 0SP