The pragmatic design of this intervention, combined with integration of the intervention into workflow, may enhance feasibility, sustainability and generalisability.
The built-in mixed methods study (multiple case analysis) will lead to new knowledge about implementation, which may be transferrable to other in-hospital interventions.
Due to the pragmatic nature of the study, participants, researchers and those conducting the intervention will not be blinded to the intervention.
Generalisability to other settings such as community and long-term care will require further exploration.
Concerns about adverse effects of medications is a top health priority among older Canadians.1 2 Polypharmacy and inappropriate medication use in older adults is common and associated with a number of harms including adverse drug reactions, falls, hospitalisations, reduced quality of life and mortality.3–6 There is particular concern about medications with anticholinergic and sedative effects; older adults may be sensitive to adverse effects of these agents due to changes in pharmacokinetics (increased exposure to the drug) and pharmacodynamics (increased sensitivity to the effects).7–9
Anticholinergic medications are used for a variety of conditions such as allergic rhinitis, urinary incontinence and nausea/vomiting.10 Other medications, including some common antidepressants, may also have anticholinergic activity even when not central to their efficacy. Sedatives may be used short term to treat insomnia and anxiety; however, many medications produce sedation as an unintended side effect.8 11 While these medications can have therapeutic effects, their use in older adults has been linked to multiple adverse effects, manifesting as limitations in physical and cognitive function.8 Furthermore, there may be reduced or limited benefits of these agents.7 8 Taken together, in older adults with multimorbidity and polypharmacy, there is a picture of potentially reduced benefit and increased risk associated with use of anticholinergic and sedative medications.
The effects of these medications may be amplified in frail individuals. Frailty is a condition of cumulative reduction in function of multiple body systems.12 Frail individuals are vulnerable to external stressors and less able to recover; as such, they are at greater risk of medication related harms.13 Despite the knowledge of the risks associated with anticholinergic and sedative medications, their use is relatively common with studies showing use of one or more of these agents in approximately 20%–80% of older adults.8 Concerningly, their use may also be more common in frail older adults who are at further risk of harm from medication use.13 14
The Drug Burden Index (DBI) is an evidence-based pharmacological risk assessment tool developed to measure exposure to anticholinergic and sedative medications, which impair physical and cognitive function.15 An increasing DBI score has been associated in several cross-sectional studies internationally with poorer physical function, reduced quality of life, frailty, falls and hospital readmissions in older adults.8 Cognitive decline and mortality have been found to be associated with an increased DBI score in some cross-sectional studies but not others.8 16 Longitudinal studies have found that an increased DBI score is independently associated with lower physical function over 5 years, poorer delayed memory performance, increased incidence of frailty and hip fracture, physician visits and mortality.14 17 These results represent 20 different studies, span multiple countries (Australia, Canada, Finland, the Netherlands, New Zealand, UK and USA) and provide a substantial argument to reduce DBI score in individuals where possible.8
Optimising medication use and reducing exposure to potentially harmful medications through deprescribing (supervised withdrawal of inappropriate medications) may improve outcomes in older adults. Even so, there are numerous barriers to deprescribing such as lack of recognition of potentially harmful medications and limited time of clinicians.18 Hospitalisation provides a unique opportunity to initiate deprescribing as there is access to an interdisciplinary team, which can conduct short-term monitoring in a controlled environment.19 Pharmacists, in their role as medication experts on the interdisciplinary hospital team, are well positioned to assess medication regimens of hospitalised older adults.19 As such they can lead medication optimisation strategies, including deprescribing.20
Over 40 different tools have been developed to assess the appropriateness of prescribing and therefore could have potential use in interventions to optimise medication use.21 Given the known association of DBI score and patient harm, the DBI has been proposed as an innovative tool to identify older adults at high risk of medication associated harm and to highlight medications that may be suitable for deprescribing.15 The DBI was chosen for this study due to the extensive external validation against clinical outcomes.21 It is also the only tool that takes into account the specific dose taken by the patient.21 Additionally, rather than just highlighting specific medications that are high risk in older adults, it considers the cumulative risk of multiple medications and their doses and provides a score such that the aim can be to reduce the score, rather than stopping specific medications.22
The DBI Calculator was developed and validated to automatically calculate the DBI score and produce a detailed report with suggestions to improve the medication regimen of a patient.22 In a previous study, 80% of pharmacists found use of the DBI Calculator an accurate and feasible addition to their practice.22 As many older patients in hospital are prescribed large quantities of medications, tools such as the DBI Calculator, may assist pharmacists in targeting deprescribing efforts.23 24
The aims of this study are to: (1) determine the effect of integration of an electronic clinical decision support tool (the DBI Calculator) into pharmacist medication optimisation activities on medication changes and clinical outcomes, (2) assess whether there is a variable effect of the intervention based on participant characteristics (ie, frailty status and sex) and/or the setting of the intervention (ie, different ward and/or hospital characteristics) and (3) explore implementation of the DBI Calculator into pharmacist-led medication optimisation activities during inpatient admissions.
This project is an in-hospital prospective interventional implementation study across four sites and hospital settings in Nova Scotia, Canada. The study consists of a preintervention control cohort that involves a retrospective chart review of patients discharged from the same ward as the intervention over 1 week prior to intervention start and will be used for comparison with the intervention group. A before/after intervention method was chosen as a pragmatic method to both determine the outcomes of the intervention and explore implementation. Additionally, this method was chosen to minimise contamination bias. A multiple case study (a substudy of the before/after intervention) will also be used to explore the process of implementation and identify factors that shaped implementation.
Participants will be identified and included as per inclusion and exclusion criteria below if they are admitted to one of the target wards (including both planned and unplanned admissions) during the relevant period for the intervention and control groups. Participants who die during admission will be removed from the study. Those who are transferred to another ward will be considered ‘discharged’ from the ward (but included in the intention to treat analysis).
At each ward, the intervention period will last for 2 months, with training on use of the DBI Calculator provided to the ward pharmacist in the week prior to the intervention period. Participants admitted to an intervention ward during the study period will be screened by an in-hospital clinical staff member (ward nurses and/or pharmacists). Screening will involve checking whether the participant fulfils the inclusion/exclusion criteria. If the patient is eligible for inclusion, the clinical staff will ask potential participants or a substitute decision maker (where appropriate) if they are willing to have a researcher approach them to discuss participation. The staff will then notify the research team member who will proceed with the informed consent process and confirm eligibility.
Identification of participants for the retrospective group admitted to one of the target wards will be conducted via screening of electronic medical records of patients discharged from the target ward at least 1 week before the start of the intervention. That is, participants will be those eligible for inclusion consecutively discharged in reverse order, working back in time from 1 week prior to the start of the intervention until the desired sample size is achieved.
Age ≥70 years old.
DBI score >0: taking ≥1 regular medication with a sedative or anticholinergic effect prior to admission to the ward (ie, medications taken at home prior to admission or on transfer from another ward).
Admitted to study ward ≤7 days ago (from home or transfer).
Written informed consent able to be obtained from participant or substitute decision maker.
Able to communicate in English.
Expected discharge within 24 hours of recruitment or 48 hours of admission.
Terminal phase of illness (expected to die during current admission) OR noted to be ‘palliative care’ (written in progress notes or consultation by the palliative care team).
Usual residence outside Nova Scotia.
Currently enrolled in another research study that involves administration of an experimental medication or a medication not approved by Health Canada.
The intervention is multifaceted and involves a pharmacist-led medication optimisation initiative using an electronic decision support tool (the DBI Calculator). The DBI Calculator acts as a mechanism for pharmacists to identify and review potentially harmful anticholinergic and sedative medications. The intervention includes calculation of the DBI score and the DBI report that also acts as a communication and documentation tool both in hospital and on discharge. Provision of information to the participant or substitute decision maker on discharge is also standardised as part of the intervention. An overview of the study flow is shown in figure 1 and further outlined below.
Initially, the ward pharmacist will calculate a DBI score from the admission medication list or transfer medication reconciliation list, and a DBI report will be created. The admission medication list is referred to as a Best Possible Medication History (BPMH). A BPMH is a complete list of all medications (prescription and non-prescription, regular and when required) taken by the patient prior to admission to hospital. In the study hospitals, the BPMH is conducted by pharmacist or appropriately trained pharmacy resident, registered pharmacy technician, pharmacy practice assistant, physician, nurse or nurse practitioner. Regardless of who completed the BPMH, the intervention was delivered by the pharmacist. After creating the DBI report, the pharmacist will discuss their recommendations (using the report) with the healthcare team and then the participant/family as appropriate. All decisions about medication changes will be conducted as deemed appropriate by the healthcare team as would occur in regular care (and will not be dictated by the research team). On discharge, the DBI report and a letter about the study will be sent to the participant’s family doctor (although no specific actions are required from them as a part of the study). If the participant has identified a regular pharmacy, a copy of the cover letter and DBI report will also be faxed to the pharmacy. A discharge medication list in the form of a medication calendar with details of changes made during admission will be provided to the participant/family prior to their discharge. A medication calendar is a list of the patients’ medications and indication for each medication. The dose and frequency of each medication is noted in a morning, noon, supper and bedtime format resembling a calendar. The pharmacist will provide verbal information to the participant or substitute decision maker about the changes including information as to why the changes were made, whether there is any monitoring required (ie, follow-up with their doctor or symptoms to monitor) and if they should continue tapering of their medication following discharge.
The pharmacist will be instructed to incorporate use of the DBI Calculator into usual care for the intervention group. In usual care (ie, care received by the retrospective control group), patients have a BPMH conducted, medication reconciliation and review (by pharmacist, pharmacy resident or nurse) and sending of a discharge summary of treatment decisions to the participant’s family doctor. In usual care, provision of verbal and/or written information to inpatients on discharge is variable and at the discretion of the pharmacist (can include verbal counselling, medication calendars and/or commercial handouts). While it is possible for healthcare professionals other than pharmacists to complete certain medication-related activities in hospital (such as nurses completing the BPMH) in both the intervention and control groups, this study will examine the addition of a pharmacist-led intervention to usual care (integrated into regular activities where possible).
The DBI Calculator is a web-based clinical decision support tool. The user (pharmacist) creates a new participant profile in the software and enters the participant’s medication regimen/list (including doses and frequencies) as per the BPMH or transfer medication reconciliation. The medication list includes all prescription medications (regular and as needed (PRN: Pro Re Nata)) and can include over-the-counter medications. Medications with non-oral routes of administration (eg, patches and eye-drops) are also included. Within the DBI Calculator, there is a section to enter suspected sedative and anticholinergic adverse effects that the participant is experiencing. Additionally, for each medication entered, a free-text recommendation can be added, and an action (continue as clinically indicated, reduce dose, cease, no change, increase the dose, reduce dose with plan to cease or therapeutic substitution) can be assigned. Once this information is entered, a ‘DBI Report’ is created that includes all entered information plus the participant’s DBI score with supporting information about the DBI. Ward pharmacists will be provided with education on using the DBI Calculator by a member of the research team prior to the start of the study.
The DBI for every participant is calculated using the equation,
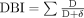
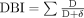
where D is the daily dose and δ is the minimum adult licenced daily dose, as a surrogate for the dose required for 50% of the maximal effect. For this study, the minimum adult licenced daily dose was extracted from the product monograph in the Health Canada Drug Product Database if available and otherwise verified using a second Canadian drug information resource (Compendium of Pharmaceuticals and Specialties).25 26 A score from 0 to 1 is calculated for each drug ingredient with anticholinergic and/or sedative effects based on the dose taken.8 15 16 Medications with both anticholinergic and sedative effects were classified as anticholinergic. Complementary medications and medications that were prescribed ‘when required’ were excluded from the calculation. A difference in DBI of 0.5 is associated with clinically significant differences in physical function and falls.8 15
The intervention is being investigated within hospital wards in Nova Scotia, Canada. The four wards, from three different hospitals, are purposely chosen to represent a variety of settings/contexts such as ward type and size of the hospital. The four sites are as follows: (1) geriatric ward in a large tertiary care centre in an urban setting, (2) surgical ward in a large tertiary care centre in an urban setting, (3) mixed general internal medicine and surgical ward in an urban small community hospital and (4) general internal medicine ward in a rural small community hospital.
See table 1 for details on how outcomes were assessed.
Data collection and source of data
Proportion of inpatients in whom DBI score is decreased, unchanged or increased at 3 months after discharge, compared with at the time of hospital discharge.
Total number of medications at hospital discharge and at 3 months after discharge.
Proportion of inpatients who experience a clinical outcome during hospitalisation.
Proportion of inpatients who had a clinical outcome within 3 months of discharge
Mean time (or median as appropriate) taken by clinical pharmacists to integrate the DBI Calculator into regular activities per participant.
Exploration of the process of implementation and identification of factors that shaped implementation (outcome of mixed methods analysis).
Participants will be recruited, and baseline data will be collected during admission. Data collected and method of collection for the preintervention, intervention and 3-month follow-up are outlined in table 1. In addition to the data collected to assess outcomes (medications, adverse drug reactions, falls, pressure ulcers, emergency department visits, rehospitalisation and mortality), data will be collected to describe the participant sample and explore factors that might influence the process of implementation (eg, sociodemographic data, frailty status, comorbidities, reason for admission, length of hospitalisation, patient attitudes towards deprescribing, quality of life and cognition).
Basic details about the intervention implementation process will also be captured. Specifically, we will capture whether the DBI report was created and placed in the participant’s chart, whether a copy of the DBI report was sent to the participant’s family doctor and/or pharmacy and whether the pharmacist provided written and oral information on discharge. Where these elements are not conducted, a reason why will be sought where possible (ie, unexpected discharge).
The power calculation is based on preliminary results of an in-hospital, randomised controlled trial of the DBI Calculator conducted in Australia.27 To detect a significant difference in proportion of participants who had a reduction of their DBI score by ≥0.5 points (a difference of 0.5 points is associated with clinically significant differences in physical function and falls),8 15 26 participants are required per group per site (alpha=0.05, power=0.8). To detect a difference between adverse drug reactions found in the Australian study, a total of 157 participants are required in each group. Therefore, we aim to recruit 40 intervention participants per site: 160 intervention participants across all sites (and collect data for the same number of participants in the preintervention groups) to detect a change in prescribing at each site and a change in in hospital adverse drug reactions across all sites. To allow for dropouts, those transferred to different units during the study and missing follow-up data, we aim to recruit up to 50 participants per ward. Requests for the estimates used for sample size calculations, which are based on currently unpublished data, will be considered by the authors on a case-by-case basis.
The difference in the primary outcome (proportion of participants with a reduction of ≥0.5 in their DBI score between admission and discharge) between control and intervention groups will be analysed using a χ2 test. Secondary clinical outcomes (proportion of participants with new in-hospital adverse drug reactions, falls and pressure ulcers and one or more emergency department visits, rehospitalisation and mortality in the 3 months after discharge) will also be analysed using a χ2 test. Change in number of medications during admission between control and intervention groups will be analysed using appropriate parametric or non-parametric tests for continuous data depending on distribution of the data. Time taken by pharmacists to use the tool will be presented descriptively.
The relationship between DBI score following discharge and hospital readmission or mortality will be explored by unadjusted and adjusted (for age, sex and site) regression methods. In addition, other characteristics associated with the DBI score at baseline will be explored and considered in adjusted regression models.
Analyses will be conducted as intention to treat, with missing data imputed using multiple imputation methods.28 29 Statistical significance is set at p<0.05. Each participant will be assigned a number prior to data entry to maintain anonymity.
A subanalysis to determine if there is a differential effect of the intervention based on frailty and sex will also be conducted.
To explore the process of implementation and identify elements that influenced implementation, a multiple case study (substudy) will be conducted.30 31 The assumption of this study is that multiple factors (elements) will influence the success of implementation such as setting, clinician and participant factors. The data collected for this portion includes progress notes/information contained in the participant chart, medication information and interviews with the participant/family, ward pharmacist and other members of the medical team.
All participants in the intervention study will be informed that they may be invited to participate in a substudy that involves a follow-up telephone interview (within consent for main study). At each site, participants will be purposely sampled during the final 4 weeks of the intervention period: one who had a reduction in their DBI score and one who did not (or otherwise suitable participants to explore the success of implementation). Participants will also be asked at the time of consent whether they have a family member/friend who is involved with decisions about medications. If yes, the participant will be instructed to ask this person whether they are willing to be involved in the substudy, and if agreeing, will provide contact details of the individual to the researcher. The researcher will then phone the individual to gain informed consent.
Prior to beginning the intervention on the ward, the pharmacist and relevant staff members will be provided with information about the project, including the possibility of being asked to participate in this substudy. The pharmacist delivering the intervention and members of the medical team involved in the participant’s care will be asked to provide informed consent for participation in this substudy where relevant for consented participants.
For the patient and family participants recruited for the multiple case study, it is anticipated that interviews will take approximately 30–60 min and will be conducted via phone at a time suitable to the participants. Interviews with medical team members will take approximately 15–30 min and conducted at a time suitable for them (in person or via phone with corresponding consent). For those who were involved with the care of more than one of the participants from the ward recruited for the multiple case study, they will only be interviewed once. Interviews will be audio recorded (then transcribed) and will be semistructured following an interview guide. NVivo will be used to manage data during collection and analysis.30 31
Using a triangulation design model, the quantitative (intervention results) and qualitative (interviews) data will be collected simultaneously and analysed separately, after which the two sets of results will be compared looking for consistency or contrast among the results.32 This analysis will address the third aim of this study and inform the secondary outcome of exploration of the process of implementation and identification of factors that shaped implementation. A logic model describing the potential relationship between the intervention, determinants and outcomes has been developed a priori and will guide this analysis (figure 2).
A community representative has been engaged as a member of the research team and has provided input for several aspects of the study including confirmation of the research question relevance, participant recruitment and information, clinical outcomes and planning for dissemination of results. To ensure the community representative is engaged and supported in this role, a meeting with the project lead and the Patient Engagement Coordinator of the Maritime SPOR SUPPORT Unit and regular meetings with the project lead were held. At the conclusion of the project, a lay summary of the project and findings will be prepared for the general community audience, which will include community representative input and will be placed on the provincial health authority public website.
This study provides an electronic decision support tool and a process to enhance the activities of ward pharmacists that may lead to improved clinical outcomes for older adults.8 15 19 22 All changes being made to participants’ medications (or any other aspects of their care) will be done by their medical team (with no control by the research team). The potential harms of medication discontinuation have been recently reviewed and include adverse drug withdrawal reactions and disruption of the doctor–patient relationship; however, the authors concluded that the potential for these harms was low when planned in conjunction with healthcare professional(s) and patient/family members and monitored.33 Any adverse effects of changes to medications will be monitored and documented by the medical team as part of the standard of care.
To reach researchers and healthcare professionals, traditional methods of dissemination will be used including peer-reviewed manuscripts and presentations at conferences and continuing education sessions.
Balancing the risks and benefits of medication use in older adults with multimorbidity is challenging. With more than half of older adults taking one or more unnecessary medications, reduction of polypharmacy and potentially inappropriate medications is imperative. Evidence on how best to execute and implement deprescribing strategies, including usefulness of clinical decision support tools to facilitate the process are emerging. However, the best method of deprescribing and the effect on clinical outcomes is still not clear. Deprescribing medications can be difficult due to barriers such as prescribing inertia, lack of process and feasibility. Despite this, 90% of patients report they are willing to have a medication deprescribed.34 Some of the most successful deprescribing initiatives described in the literature have incorporated clinical pharmacists and targeted specific medications.35 36
While we have designed a pragmatic study with the aim of integration into regular clinical activities to determine success of implementation and sustainability, this study has several limitations. Due to limitations of the DBI Calculator, the large number and variability of products available, and data sources, we are not able to include and collect information on all over-the-counter (non-prescription), herbal/natural health products and complementary medicines. Also, a 3-month follow-up may not be sufficient to determine long-term effects of the intervention. The DBI Calculator is currently a stand-alone program and as such integration with existing information technology systems and programme, such as prescribing software, is likely required for long-term sustainability. Due to the pragmatic nature of the study, participants, researchers and those conducting the intervention will not be blinded to the intervention. Additionally, due to the informed consent process required for the intervention participants, it is possible that older adults who are resistant to medication changes may self-select out of the study (even though willingness to have a medication deprescribed is not a requirement for inclusion). This type of self-selection will not occur in the control group as it is a retrospective sample (waiver of consent); this difference in consent processes between the intervention and control groups could therefore bias the results. As the control group data are collected retrospectively, only data that are routinely collected and documented during hospital admission can be included without possibility for clarification or checking with the participant. Finally, while this is an interventional study with a control group, there are possible factors that may confound the results. For example, change in season and change in medical and other staff between the control and the intervention periods.
This manuscript is based on trial protocol V.2.0 dated 9 November 2018. The pharmacist-led intervention to improve medication use in older inpatients using the DBI trial, opened to recruitment in February 2019 and is due to end in September 2019. Three-month outcome data will be collected retrospectively when mortality data become available through Health Data Nova Scotia (there is a lag in time between the relevant outcome period and when the data is available to be extracted). As of January 2020, we have recruited 45 intervention participants. Any protocol modifications will be communicated to relevant parties.
We would like to acknowledge the ongoing support provided by the clinical and research teams from Nova Scotia Health Authority (NSHA) Geriatric Medicine Research, Pharmacy Department and participating sites within NSHA Central Zone. We would also like to acknowledge Dr Colin Van Zoost for his input regarding the concept and design of the study and providing feedback on the presentation of the DBI Report. Lastly, we would like to thank our community representative, Marilyn Peers, for her provision of the patient, family and caregiver perspective, input regarding development of multiple case study interview guides and review of the protocol.
E-mail:
Tel: +44 (0) 141 946 6482
Address: Healthcare Skills Training International Ltd
West of Scotland Science Park
Block 7, Kelvin Campus
Glasgow G20 0SP