 FOLLOWING the revisions to the classification of medical devices under the new In-Vitro Diagnostic Regulation (May 2017) 92 per cent of IVDD manufacturers now require the services of a Notified Body, where previously only approximately eight per cent were subject to this compliance.
FOLLOWING the revisions to the classification of medical devices under the new In-Vitro Diagnostic Regulation (May 2017) 92 per cent of IVDD manufacturers now require the services of a Notified Body, where previously only approximately eight per cent were subject to this compliance.
Are you one of the manufacturers affected by this change?
If so, then you will be aware of the time pressures on all stakeholders – i.e. the manufacturer, the Notified Body and the Competent Authority.
Currently, the Notified Bodies’ cannot apply for designation until six months after Entry into Force.
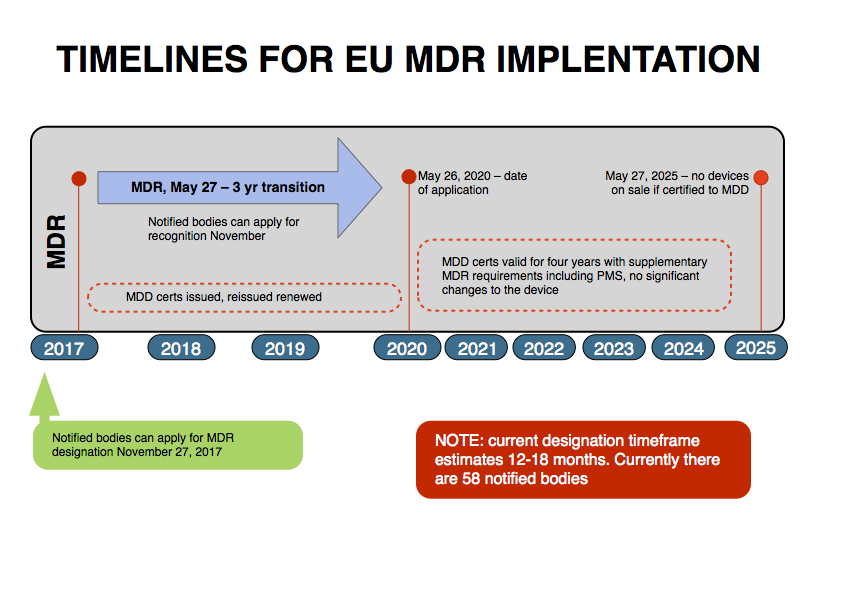
The designation process is currently estimated at 12 to 18 months, and with approximately 58 Notified Bodies, it is not possible for all Notified Bodies’ to be designated at once.
So, for those of you who have never used the services of a Notified Body, you will need to determine your strategy on how you are going to select one, based on wide criteria.
And get your plans under way now!
As you consider your regulatory strategy (a requirement of the IVDR) it is important you take account of the timeframes, together with the expiry dates of your certificates both for Quality Management System and European Conformity (CE) marking.
Individual device product life cycles will identify the additional work required to construct technical files/design dossiers to meet the specifications of the IVDR.
If you manufacture IVDD’s this affects YOU! We know the clock is ticking.
We have covered the fact that time is running down on this issue here, here, here and here.
E-mail:
Tel: +44 (0) 141 946 6482
Address: Healthcare Skills Training International Ltd
West of Scotland Science Park
Block 7, Kelvin Campus
Glasgow G20 0SP