
WE hope by now, given that the MDR & IVDR were both published in the Official Journal of the European (OJEU) on May 5, 2017, and their “Entry into Force” was 20 days later (see Article 120 – Transitional Provisions/Article 123 – Entry into Force & date of application), that you have all had time to at least scan the document.
If not, you are strongly encourage you to do so now.
You could also listen to any of the free or paid-for webinars being offered by a variety of organisations. This will also help you develop your regulatory strategy, transition planning, training plans etc.
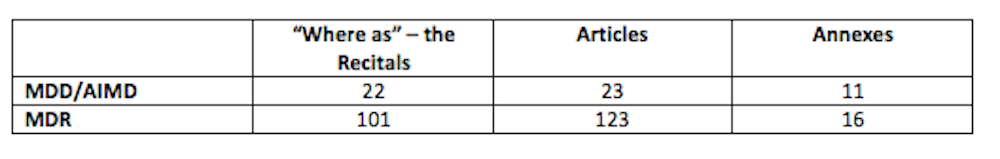
With regards to the MDD, AIMD & IVDD, there is a similar structure, insomuch as the “Recitals”, contain the “Where as” statements in the Articles and Annexes.
Having an appreciation of the structure will help you as you begin to navigate your way through the document.
Here are some simple statistics in comparing the MDD/AIMD with the MDR:
We all have some work to do. These changes will affect us all; our business and our medical devices, some of us more than others, depending on Classification of our products and other factors.
As you begin to think about transition planning, please remember we also have ISO 13485:2016 running in parallel. Its transition period is due to end on February 28, 2019.
It would also be helpful to think about the product life cycle – particularly in the context of audit timing and certification expiration dates, which are referenced in the two Articles (120, 123) as noted above.
Careful and detailed planning is required. The challenges impact on manufacturers, Notified Bodies and the UK’s Competent Authority ie MHRA.
It is important to engage with your Notified Body/Certification Agency in order to also understand their timescales.
Although the Regulations have been published, there are still many unanswered questions, including some of the legislation ie Implementing & Delegating Acts, one of which will detail requirements for UDI (Unique Device Identification).
Please be aware your NB cannot apply for designation under the MDR until six months after Entry into Force – November 27, 2017, a process which may take some time to execute before the NB is designated and able to audit.
Till next time, good luck.! Enjoy reading the MDR or IVDR.
back
E-mail:
Tel: +44 (0) 141 946 6482
Address: Healthcare Skills Training International Ltd
West of Scotland Science Park
Block 7, Kelvin Campus
Glasgow G20 0SP